However, catalysts, including those used to “split” water, have either worked well but are expensive and unstable, or are affordable and stable, but don’t work as well. Now, researchers report in ACS Central Science a new catalyst that is really the best of both worlds.
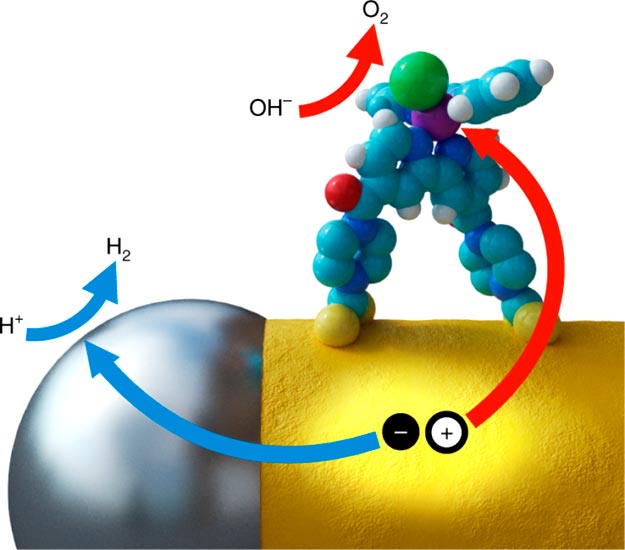
Identifying ideal materials that can split water is a long-standing problem in renewable energy storage. Catalysts, which help reactions occur, are often used in this process. “Homogeneous” ones dissolve into the reaction solution and are usually active and selective. However, they don’t work well in some applications because they are unstable and expensive. In contrast, “heterogeneous” catalysts are solids that are stable, recyclable and convenient to work with, but they are usually not very active or selective. Dunwei Wang and colleagues proposed they could get closer to the ideal catalyst by producing a hybrid material.
The researchers developed a new hybrid catalyst made of iridium dinuclear heterogeneous catalysts (DHCs) attached to a tungsten oxide substrate. They found that attaching the ends of the DHC molecules — instead of the sides — allowed the catalyst to perform optimally. The researchers suggest that this first-of-its-kind material could be an important step toward alternative solar energy storage or artificial photosynthesis.
Source: https://www.eurekalert.org
Dear User/Visitor! Please, answer on our questions: tick off one of the positions – your answer will make us able to improve our site and make it more interesting and useful!